Marine CCS (Carbon Capture and Storage) to the ocean potentially could be done in two ways: by injecting and dissolving CO2 into the water column (typically below 1,000 meters) via a fixed pipeline or a moving ship, or by depositing it via a fixed pipeline or an offshore platform onto the sea floor at depths below 3,000 m, where CO2 is denser than water and is expected to form a “lake” that would delay dissolution of CO2 into the surrounding environment (see Figure 1 below). Ocean storage and its ecological impacts are still in the research phase.
The dissolved and dispersed CO2 would become part of the global carbon cycle and eventually equilibrate with the CO2 in the atmosphere. In laboratory experiments, small-scale ocean experiments and model simulations, the technologies and associated physical and chemical phenomena, which include, notably, increases in acidity (lower pH) and their effect on marine ecosystems, have been studied for a range of ocean storage options. [Increased pH levels and ocean acidity would have disastrous effect on marine life]
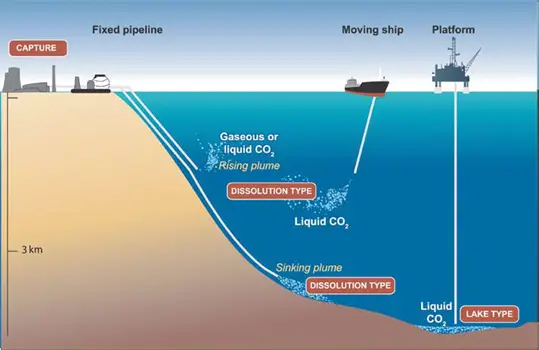
Figure 1. Overview of ocean storage concepts. In “dissolution type” ocean storage, the CO2 rapidly dissolves in the ocean water, whereas in “lake type” ocean storage, the CO2 is initially a liquid on the sea floor (Courtesy CO2CRC).
Marine CCS and adding CO2 to the ocean or forming pools of liquid CO2 on the ocean floor at industrial scales will alter the local chemical environment. Experiments have shown that sustained high concentrations of CO2 would cause mortality of ocean organisms. CO2 effects on marine organisms will have ecosystem consequences. The chronic effects of direct CO2 injection into the ocean on ecosystems over large ocean areas and long time scales have not yet been studied.
Model simulations, assuming a release from seven locations at an ocean depth of 3,000 m, where ocean storage provides 10% of the mitigation effort for stabilization at 550 ppmv CO2, resulted in acidity increases (pH decrease >0.4) over approximately 1% of the ocean volume. For comparison purposes: in such a stabilization case without ocean storage, a pH decrease >0.25 relative to pre-industrial levels at the entire ocean surface can be expected. A 0.2 to 0.4 pH decrease is significantly greater than pre-industrial variations in average ocean acidity. At these levels of pH change, some effects have been found in organisms that live near the ocean’s surface, but chronic effects have not yet been studied.
A better understanding of these impacts is required before a comprehensive risk assessment can be accomplished. There is no known mechanism for the sudden or catastrophic release of stored CO2 from the ocean to the atmosphere. Gradual release is discussed in SPM paragraph 26. Conversion of molecular CO2 to bicarbonates or hydrates before or during CO2 release would reduce the pH effects and enhance the retention of CO2 in the ocean, but this would also increase the costs and other environmental impacts.
Marine CCS material sourced from: IPCC Special Report, 2007. Carbon Dioxide Capture and Storage, Summary for Policymakers, A Special Report of Working Group III of the Intergovernmental Panel on Climate Change, pg14.
